Service Items
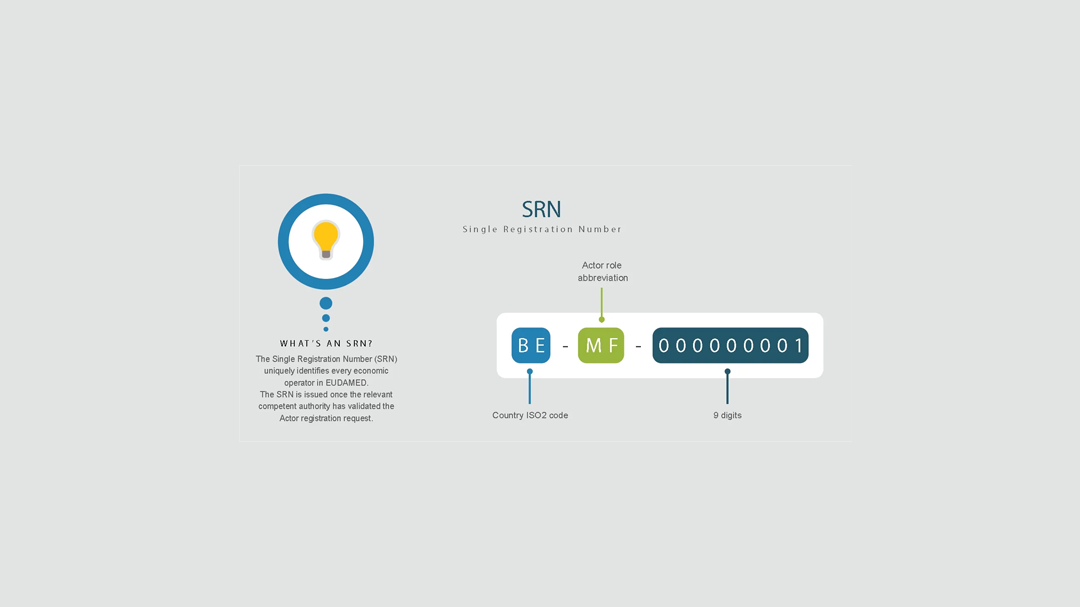
SRN registration
Variant strain test
Cmdcas certification
Medical instrument registration certificate
CSDT注册
TGA registration
CTDA registration
Mdsap certification
ISO9001 certification
ISO13485 certification
EU free sale certificate (FSC)
Mdel registration
Medical device production license
Business license of medical devices
Qsr820 certification
FDA510(K) certification
Preparation of ukca technical documents
UKCA认证
MHRA registration
UK authorized representative
Preparation of MDR CE technical documents
Study on the applicability of non professionals
Clinical trial
EU HSC Common List
MDR CE certification
FDA certification
IVDR CE certification
IVDD CE certification
EU authorized representative
IVDEAR (Shenzhen) Medical Techno
Ivdear (Shenzhen) Medical Technology Co., Ltd., subordinate to Guorui Zhongan group, is a holding subsidiary of Guorui Zhongan group. Ivdear specializes in customized regulations and registration consulting services for medical devices and in vitro diagnostic products, and provides training services for regulations and standards. Specific service areas include: EU CE certification consulting for medical devices (IVDD, MDR, IVDR), clinical experiment, ISO13485 system certification consulting, FDA registration consulting (including 510k, QSR 820, etc.) Product inspection and rectification consultation (electromagnetic compatibility, electrical safety, performance, biocompatibility, wireless, packaging test, etc.); Domestic registered nmpa consulting services (clinical scheme cro consulting services, product registration certificate consulting services, production license consulting services, import registration consulting services).
Press Center
- 2022-08-02
- 2022-08-01
- 2022-08-01
- 2022-07-26



















.jpg)

































































.jpg)